Which Equation Is Used to Describe Reaction Rates
ES E P From 2 the second term equals zero so we are left with. Therefore if the initial reaction rate is measured the only unknowns in the rate law are the rate constant k and the exponents a and b.

Rate Law And Reaction Order Video Khan Academy
V k A a B b P p where k is the rate constant A and B are reactants and P is the product with stoichiometric coefficients a b p respectively.

. What is the equilibrium expression for 2NO Br2 2NOBr. Rate - The general form of the rate law for this reaction can be written as follows. For the general reaction aAbB C aA bB C with no intermediate steps in its reaction mechanism meaning that it is an elementary reaction the rate law is given by.
Rate of reaction is the speed of a reaction. By doing experiments involving a reaction between Aand B you would find that the rate of the reaction was related to the concentrations of Aand Bin this way. Where k rate constant of the reaction A Arrhenius Constant Ea Activation Energy for the reaction in Joules mol 1 R Universal Gas Constant T Temperature in absolute scale in kelvins.
The rate law for a chemical reaction is an equation that relates the reaction rate with the concentrations or partial pressures of the reactants. The rate of a reaction is usually observed by watching the disappearance of a reactant or the appearance of a product within a given time period. ES We want to describe V o in measurable quantities but ES is not easy to measure.
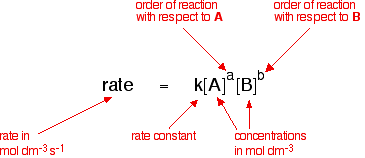
R k A a B b. Then the rate will have units like M s -1. In general the rate of a reaction v is described by an equation such as the following.
Rate of formation of ES Rate of breakdown. It is calculated using the following formula. Which of the following equations is used to describe reaction rates.
This is called the rate equationfor the reaction. DeltaH - TdeltaS a. A2B 3C A 2 B 3 C.
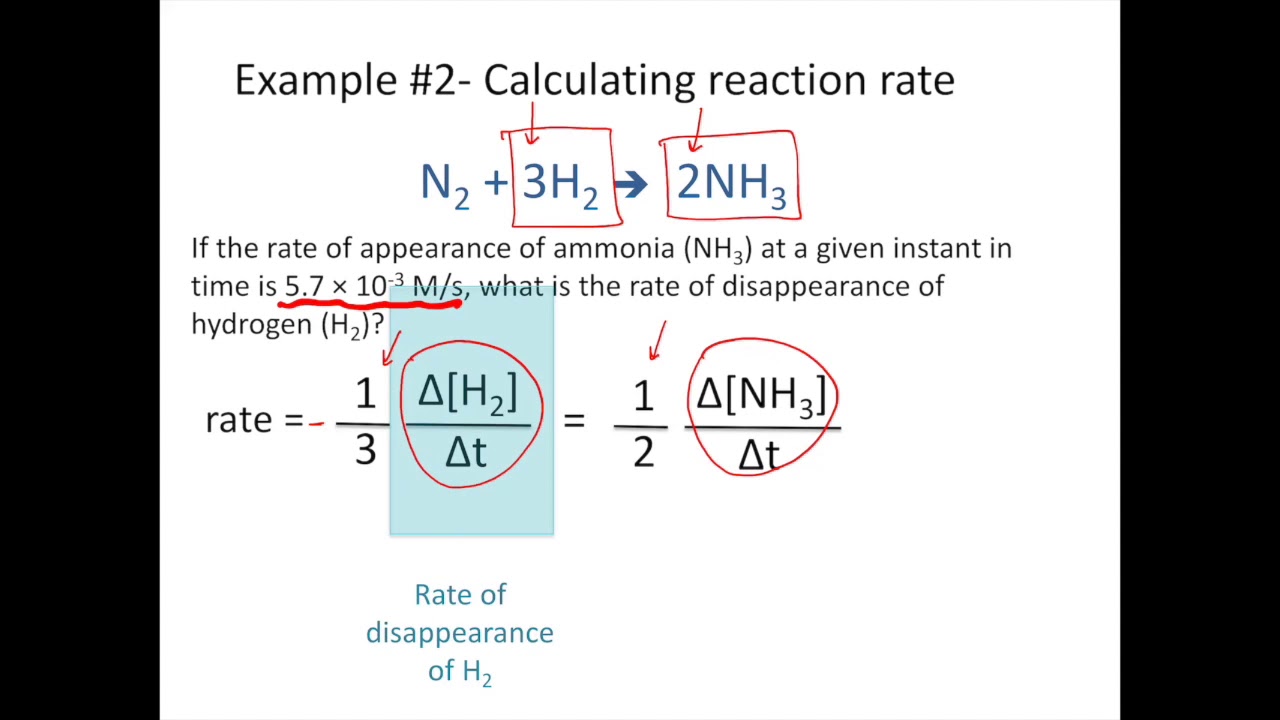
2NH3g N2g 3H2g 2 NH 3 g N 2 g 3 H 2 g The stoichiometric factors derived from this equation may be used to relate reaction rates in the same manner that they are used to related reactant and product amounts. Rate of reaction of A increase in concentrationtime interval -Δ AΔt Note that the rate is always expressed as a positive number thats the reason for the negative sign in front of the Δ A. The initial concentrations of A and B are known.
If A is decreasing at the rate of 01 molL¹min¹ B is increasing at the rate of 02 molL¹min¹. Delta reactant or productdeltaTime b. 1214 d M A d t M ˆ Ai M ˆ Ao R A Therefore the rate of reaction RA can be determined if we measure the rate of change in the mass of A in the system d MA d t and the rates of flow of A into and out of the system M ˆ Ai and M ˆ Ao.
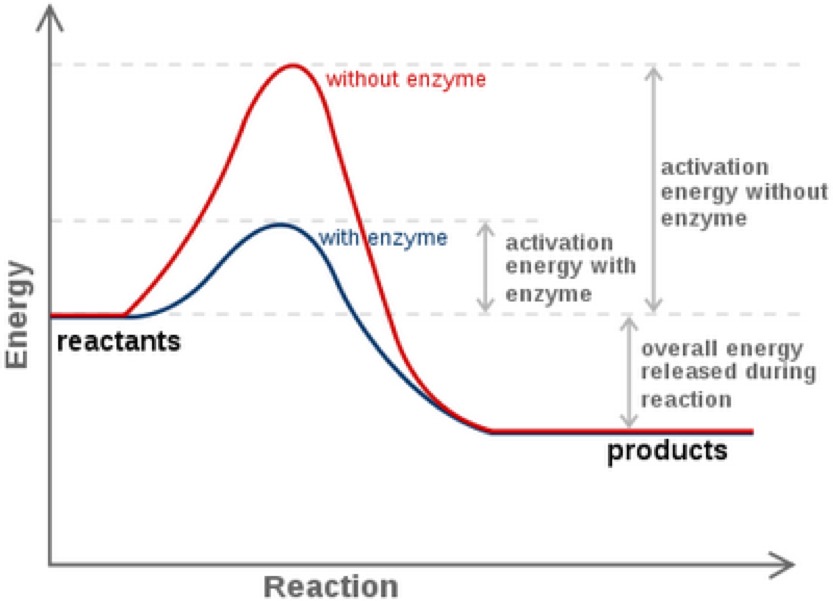
For two reactions at the same temperature the reaction with the higher activation energy has the lower rate constant and the slower rate. The equations show which compounds are produced or destroyed during the reaction and they can also be used to calculate quantities of substances present in reactions. Here the rate of appearance of product C in time interval Δt is.
So the reaction rate is normally given as change in concentration over time. Rate k cv dyexOH-y The color of crystal violet is due to the absorption of visible light at 540nm absorbance. The nature of the chemical reaction plays a large role in determining the reaction rate.
The method chosen usually depends on the reactants and products involved and how easy it is to measure changes in them. Describe the effect of concentration particle size catalysis and temperature on the rates of reactions. The concentrations of Aand Bhave to be raised to some power to show how they affect the rate of the reaction.
From 1 we know the overall rate of the reaction is determined by the rate of the catalytic step. The Arrhenius equation is used to quantify the effect of temperature on the reaction rate. The rate of consumption RC is equal to RA and RG 0.
The general rate expression can be expressed mathematically as follows. To express ES in terms of S we can start from 3. The rate of reaction usually depends on the concentration of reactants.
The most common chemical equation consists of two columns with the left. While the form of the differential rate law might be very complicated many reactions have a rate law of the following form. A chemical equation is a concise approach to depict a chemical process using symbols rather than words.
Average rate ΔC Δt average rate Δ C Δ t. Take the chemical reaction. NOBr2 NO2 Br2 b.
R kAxBy r k A x B y. Arrhenius Equation Here is the Arrhenius Equation on the temperature dependence of the rate of a chemical reaction. Consider the reaction represented by the following equation.
This is the change in concentration of a reactant or product over time. Also the two rates are different. The Arrhenius equation describes quantitatively much of what we have already discussed about reaction rates.
It is important to note that some reaction rates are negatively affected by temperature while a few are independent of temperature. The mean rate of reaction. The mass balance equation becomes.
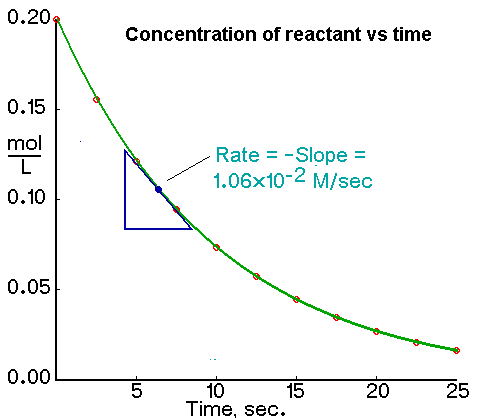
However S is known from 4. Rate of reaction amount of product formedreactant used up. No matter which quantity is measured during the course of a reaction the average rate of reaction can be calculated using the equation below.
If the change in some other property over time is used to measure the rate this property is usually converted back into concentration units.

Collision Kinetic Theory And Surface Area Foldable Rates Of Reaction Activity Chemistry Worksheets Printable Worksheets Solving Quadratic Equations

Reaction Rate Https Scienceterms Net Chemistry Reaction Rate Reaction Rate Chemistry Fun Facts

How Do I Determine Rate Of Reaction Or Kinetics Of Reaction Without Taking Into Consideration The Reactant Concentration

The Rate Law Concentration And Time Boundless Chemistry
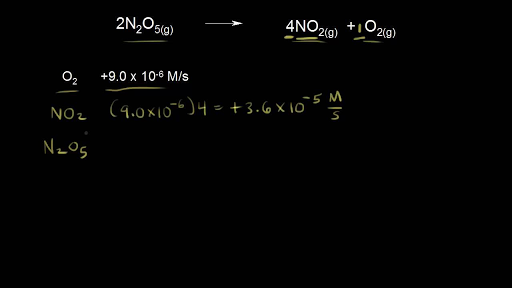
Introduction To Reaction Rates Video Khan Academy

Chemical Kinetics Reaction Rate The Change In Concentration Of Reactant Or Product Per Unit Time Chemical Kinetics Reaction Rate Gas Constant
Chemical Reaction Rates Chemistry For Majors

The Rate Law Concentration And Time Boundless Chemistry

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics Youtube

Kirstie Parker On Twitter Order Of Reaction Ozone Depletion Collision Theory

Rate Determining Step Easy Science Chemical Reactions Easy Science Exothermic Reaction

Https Www Pinterest Com Explore Le Chatelier S Principle Teaching Chemistry Chemistry Lessons Chemistry Education

How Do You Calculate The Reaction Rate A Plus Topper Howtocalculaterateofreaction Reaction Rate Chemistry Classroom Relative Atomic Mass

Comments
Post a Comment